Speak to any scientist, and they would probably confirm the truth behind the myth that science simply wouldn’t happen without the input of coffee. Most of us seem to mainline it in order to achieve anything in a day, to the point where my university coffee shop even has a sign explaining the meaning of ‘procaffeination’, which is to delay or put off doing something until you have had a cup of coffee.
Given that I have a two hour long rush-hour commute into work by bus, tube, train and walking, it is inevitable that a little of my morning grande black Americano spills out of the top and down the side of my cup now and again. What you may not realize is quite how interesting this is from a scientific perspective.
When the dregs of the coffee are drained and the cup has been disposed of, there is more than just the caffeinated buzz of productivity that remains. The coffee ring is a fascinating subject for scientists to study. It has long baffled experts as to why a coffee ring evaporates to leave a mark that is darker on the edges than the sides.
It is a truth universally acknowledged that a scientist in possession of a good cup of coffee must be in want of some good scientific discoveries (Photo credit: Tristan Fewings/Getty Images)
Coffee is a suspension of very fine, dark particles in water. A water droplet on an average tends to be a domed entity, with a tall center that gently tapers in height towards the shallower edges. As such, as a water droplet evaporates, we can see the edges of the droplet receding towards the center, as it is easier for water molecules to evaporate from these thinner edges than from the bulk of liquid water in the center. The outer water molecules evaporate and are replaced with molecules from towards the center. This evaporation has been shown to produce a pulling force or capillary flow on the molecules from the center of the droplet out towards the edges. If there are particles suspended within the water that are fine enough to be swept along with this induced flow from the center of the droplet to the edge, they are unable to evaporate away, and so accumulate at the edge of the water droplet. As the water evaporates away, we are left with the familiar ‘coffee ring’ effect of dried drops that are darker on the edges than they are in the center.
However recently scientists and engineers from Imperial College, London and the University of Edinburgh in the UK, MIT and the University of Maryland in the USA and Tianjin University of Commerce in China have through their international collaboration made some interesting headway in the understanding of the mechanism behind dark-edged dried droplets, determining that the surfaced tension of the droplet also plays a part in this by interacting with the particles suspended within the droplet. The effect of the geometry of a droplet on the coffee-ring effect has also been analysed. Most studies have been conducted on round droplets, usually in an attempt to simplify the maths behind the mechanisms, but one group of collaborators recently uncovered a law that determines that the greater the curvature of a region in a droplet, regardless of the geometry of the droplet, the greater the deposition of particles upon evaporation.
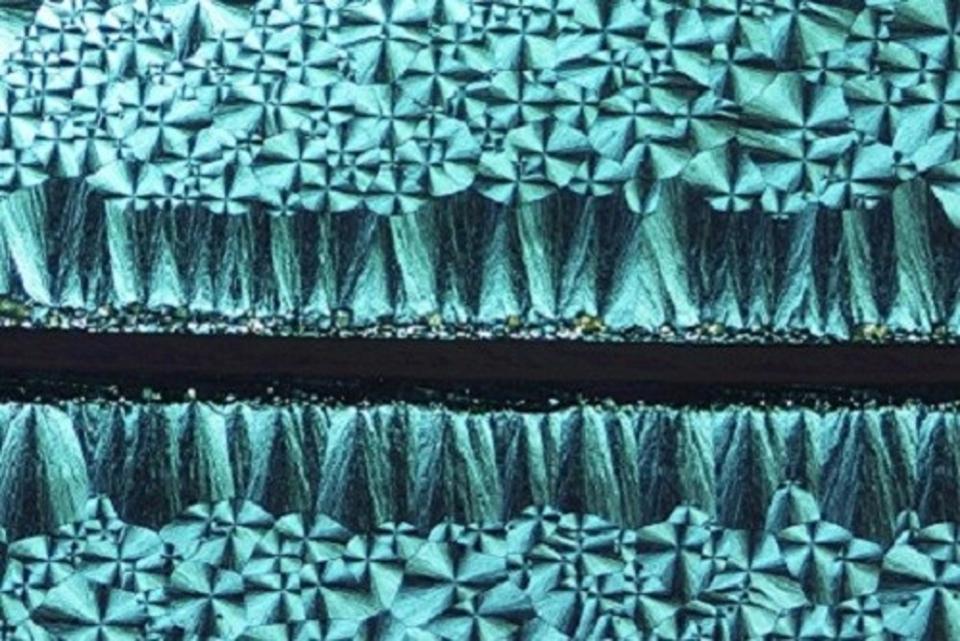
So far, so interesting, but why does this matter? Well, understanding how particles can be deposited by tailoring the shape, size and position of droplets with creating thin films using a drop-coating technique could lead to films that can either be made more uniform across a substrate, or films that vary in thickness or position in an intentional and desirable way. Methods of drop-coating that can specify where thick lines of deposition should be could lead to advances in depositing metal particles to create films of electronic circuits, and other patterned materials that can be annealed to go create other functional thin films. By varying the materials suspended in the droplets, scientists can also preferentially arrange molecules by creating molecules with the sorts of functional groups that can interact with other molecules in such a way that they line up as the droplet evaporates, generating areas of localized organisation that can give rise to greater uniformity and specific desirable properties in a range of materials. This is of particular interest to semiconductor scientists who are using this technique to arrange many tiny crystals into a useful order to increase the efficiency of their materials that can be used in everything from electronics to solar energy harvesting.
Thanks to a classic case of procaffeination and the scientific curiosity to study coffee rings close-up, these groups of scientists are uncovering the fluid dynamics behind the evaporation of droplets and using it to create bespoke materials with a range of new and useful properties.
By controlling the rate of evaporation of a solvent from a droplet, it is possible to create an ordered polycrystalline structure within a thin film. (Photo credit: KAUST 2017)